Ams 800 Urinary Control System Mri Safe
Ams 800 urinary control system mri safe. AMS 800 ARTIFICIAL URINARY SPHINCTER. AMS 800 Urinary Control System. AMS 800 Artificial Urinary Sphincter.
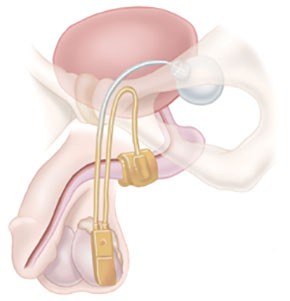
AMS 700 CX CXM CXR Ultrex. Once implanted and activated squeezing a bulb on the control pump releases the pressure exerted by a cuff that otherwise closes off the urethra. For implant procedure information consult the AMS 800 Urinary Control System Operating Room Manual.
Safety Topic Subject. Safety Topic Subject AMS 800 Urinary Control System American Medical Systems Inc. It mimics normal sphincter function by opening and closing the urethra at.
Is the AMS 800 MRI compatible. A cuff surrounds the urethra which can be filled or emptied of fluid from a pressure-regulating reservoir by the patient squeezing a pump in his scrotum. Considered the gold standard treatment for male stress urinary incontinence the AMS 800 Artificial Urinary Sphincter provides proven discreet bladder control.
Strict patient selection optimum preoperative bladder management and regular follow-up ensure low complication and high efficacy rates in the long term. The most frequent indication was following prostate surgery with the best results. AMS 800 Artificial Urinary Sphincter.
AMS 800 Artificial Urinary Sphincter Deactivate Activate Animation - YouTube. MRI-Related Heating Non-clinical testing has demonstrated the AMS 800 Urinary Control System product line produced the temperature rises during MRI performed for 15 minutes of scanning in the respective MR systems which would not pose a hazard to the human subject AMS 800 Urinary Control System. No unsafe magnetic field interactions w ere identified by this research.
The device can be scanned safely under the. Now nearly 40 years old the AMS 800 Urinary Control System by Boston Scientific is still the most widely used artificial urinary sphincter in the world.
Prior prosthesis implantation should not preclude patients from having an MRI.
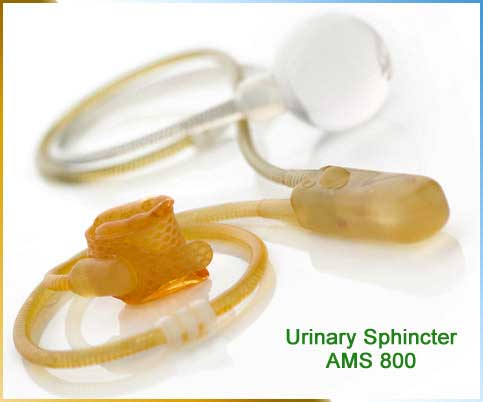
Safety Topic Subject AMS 800 Urinary Control System American Medical Systems Inc. Federal law restricts this device to sale by or on the order of a physician. Brief Device Description The AMS 800 Urinary Control System is an implantable fluid filled solid silicone elastomer device used to treat stress urinary incontinence. Non-clinical testing has demonstrated that the AMS 800 Urinary Control System product line may compromise the MR image quality if the area of interest is relatively close to the position of the implant. The most frequent indication was following prostate surgery with the best results. 1 MRI Safe The follow ing AMS product does not contain metallic components and therefore is considered MRI Safe. Considered the gold standard treatment for male stress urinary incontinence the AMS 800 Artificial Urinary Sphincter provides proven discreet bladder control. The AMS 800 Urinary Control System The AMS 800 Urinary Control System is intended for use in the treatment of male urinary incontinence. AdVance Male Sling System MRI Conditional MR Conditional Some AMS products are MRI Conditional up to 30 Tesla.
AMS 800 ARTIFICIAL URINARY SPHINCTER. AMS 800 Urinary Control System Additional Data. Considered the gold standard treatment for male stress urinary incontinence the AMS 800 Artificial Urinary Sphincter provides proven discreet bladder control. Static Magnetic Field 15 Teslaa 30 Teslab. AMS 800 Control Pumps are used in artificial urinary control systems which are implanted in patients with moderate to severe incontinence to simulate normal urinary function. Talk to your doctor for a complete listing of risks warnings and important safety. David Mills from Moorgate Andrology explains what the AMS 800 Urinary Control System is and how it worksThe AMS 800 Urinary Control System is an artificial.



























Post a Comment for "Ams 800 Urinary Control System Mri Safe"